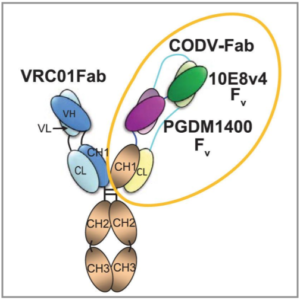
 In people with HIV, single infusions of bNAbs have demonstrated antiviral activity and can reduce plasma virus loads. However, virus variants that are resistant to the single bNAb emerge quickly and therefore limit the activity and therapeutic potential of bNAb monotherapy. SAR441236 is an engineered tri-specific bNAb produced by Sanofi that combines the CD4bs specificity of VRC01-LS, the V1/V2 glycan-directed binding of PGDM1400, and the gp41 MPER binding of 10E8v4-variant into one antibody molecule. This tri-specific bNAb neutralizes 204 of 208 (98%) viruses from a standard neutralization panel and provided 100% protection to non-human primates against intra-rectal challenge by a mixture of SHIVs, each resistant to one of the bNAb components.
In people with HIV, single infusions of bNAbs have demonstrated antiviral activity and can reduce plasma virus loads. However, virus variants that are resistant to the single bNAb emerge quickly and therefore limit the activity and therapeutic potential of bNAb monotherapy. SAR441236 is an engineered tri-specific bNAb produced by Sanofi that combines the CD4bs specificity of VRC01-LS, the V1/V2 glycan-directed binding of PGDM1400, and the gp41 MPER binding of 10E8v4-variant into one antibody molecule. This tri-specific bNAb neutralizes 204 of 208 (98%) viruses from a standard neutralization panel and provided 100% protection to non-human primates against intra-rectal challenge by a mixture of SHIVs, each resistant to one of the bNAb components.
In this collaborative multi-site project with the AIDS Clinical Trial Group and Sanofi, for which Athe is the Protocol Chair (A5377), we are conducting the first-in-human ascending dose study to determine the safety, PK and anti-HIV-1 activity of SAR441236 in participants with HIV on stable, suppressive antiretroviral therapy (ART) and in treatment-naïve participants with HIV.
This project is currently recruiting for a qualified postdoctoral candidate. The focus of this work can combine virologic and immunologic Aims that may focus on virus reservoir effects, env selection pressures, and the ability of the trispecific bNAb to modulate humoral immunity